
Battery cells store and release energy, powering devices from smartphones to electric vehicles. Understanding the different types—primary, secondary, fuel, and reserve cells—helps users select the best option for specific battery applications. Major industries like EVs, solar energy storage, consumer electronics, drones, medical devices, and industrial equipment rely on advanced battery cells for performance and efficiency.
Rapid growth in these sectors drives innovation, making battery technology a key factor in global market expansion.
Popular Applications of Our Battery Cells
-
18650 and 21700 Cells: Ideal for flashlights, power banks, electric bikes, UPS systems, and laptops
-
LiFePO4 Cells: Long life and safety for solar storage, RVs, home energy systems
-
Pouch and Prismatic Cells: Used in tablets, drones, handheld POS devices, and wearable tech
-
Coin/Button Cells: Best for watches, hearing aids, remote controls, and medical instruments
📦 Battery Cell Models We Offer (Hot-Selling)
-
INR18650-35E – Samsung 3500mAh 3.7V
-
LG MJ1 18650 – 3500mAh high-drain
-
LFP32700 – 3.2V 6000mAh LiFePO4 cell
-
Sanyo NCR18650GA – 3450mAh
-
Rechargeable CR2032 – 3V lithium coin cell
Key Takeaways
-
Battery cells come in four main types: primary (single-use), secondary (rechargeable), fuel cells, and reserve cells, each suited for different needs and devices.
-
Common battery formats include cylindrical, prismatic, pouch, and button cells, with each format offering unique advantages for space, safety, and performance.
-
Lithium-ion batteries lead the market due to their high energy density, long cycle life, and wide use in electric vehicles and electronics.
-
Choosing the right battery depends on factors like energy density, cycle life, safety, cost, and device requirements to ensure optimal performance and reliability.
-
Proper battery management and safety measures, including certified systems and recycling, help extend battery life and protect users and the environment.
Battery Cells Overview
What Is a Battery Cell
A battery cell is a compact unit that stores and delivers electrical energy. Each cell contains five main components: an anode, a cathode, an electrolyte, a separator, and current collectors. The anode, often made from metals like zinc or lithium, serves as the negative electrode and releases electrons during operation. The cathode, usually a metallic oxide, acts as the positive electrode and accepts electrons. The electrolyte sits between the electrodes and allows ions to move while blocking the flow of electrons directly. The separator keeps the anode and cathode apart to prevent short circuits but lets ions pass through. Current collectors, made from materials such as copper and aluminum foil, help transfer electrons to and from the external circuit. Together, these parts enable battery cells to convert chemical energy into electrical energy, powering a wide range of devices.
How Battery Cells Work
Battery cells operate through a series of electrochemical reactions. When a device draws power, the anode undergoes oxidation, releasing electrons and positive ions. Electrons travel through an external circuit, providing usable electricity, while ions move through the electrolyte toward the cathode. At the cathode, a reduction reaction takes place as it accepts electrons. The separator ensures that only ions, not electrons, pass between the electrodes. The voltage produced by a cell depends on the difference in electrochemical potential between the anode and cathode. The energy output is influenced by the chemical reactions inside the cell and by factors such as internal resistance and heat generation. In rechargeable battery cells, these reactions can be reversed, allowing the cell to store energy again for future use.
Tip: Battery cells come in many forms, but all rely on the same basic principles of electrochemistry to store and deliver energy efficiently.
Types of Batteries
Understanding the different types of batteries helps users select the right power source for their needs. The main classifications include primary (single-use), secondary (rechargeable), fuel cell, and reserve cells. Each type features unique chemical compositions, performance characteristics, and applications.
Primary Battery Cells
Primary battery cells are non-rechargeable batteries designed for single-use. Once depleted, users must replace them. These batteries use chemistries such as zinc-carbon, alkaline, and various lithium cells. The zinc-carbon cell uses a zinc container as the anode and a carbon rod surrounded by manganese dioxide and ammonium chloride as the cathode. This design produces an initial voltage of about 1.5 V, which decreases as the battery discharges.
Primary batteries offer several advantages:
-
Lightweight and compact
-
Excellent charge retention
-
Lower initial cost
However, they are not suited for high discharge rates or heavy load applications. The most common chemistries and their applications include:
|
Chemistry Type |
Key Advantages |
Typical Applications |
Typical Lifespan |
|---|---|---|---|
|
Leclanche (Zinc-Carbon) |
Inexpensive, reliable |
Telephones, toys, remotes, flashlights, clocks |
Several years |
|
Alkaline |
Stable capacity, good shelf life, works in sub-zero temps |
Toys, remotes, MP3 players, cameras |
Around 5 years |
|
Silver-Zinc |
Low self-discharge, higher voltage |
Watches, hearing aids, aerospace, military |
5–8 years |
|
Zinc-Air |
High energy density, excellent shelf life |
Hearing aids, watches, signaling devices |
Up to several years |
Many lithium cells, such as LiCFX, LiMnO2, and LiSOCl2, serve specialized roles. For example, LiCFX powers pacemakers due to its low weight and compatibility with titanium casings. LiMnO2 appears in consumer electronics and IoT devices, while LiSOCl2 supports extreme environments with lifespans up to 40 years.
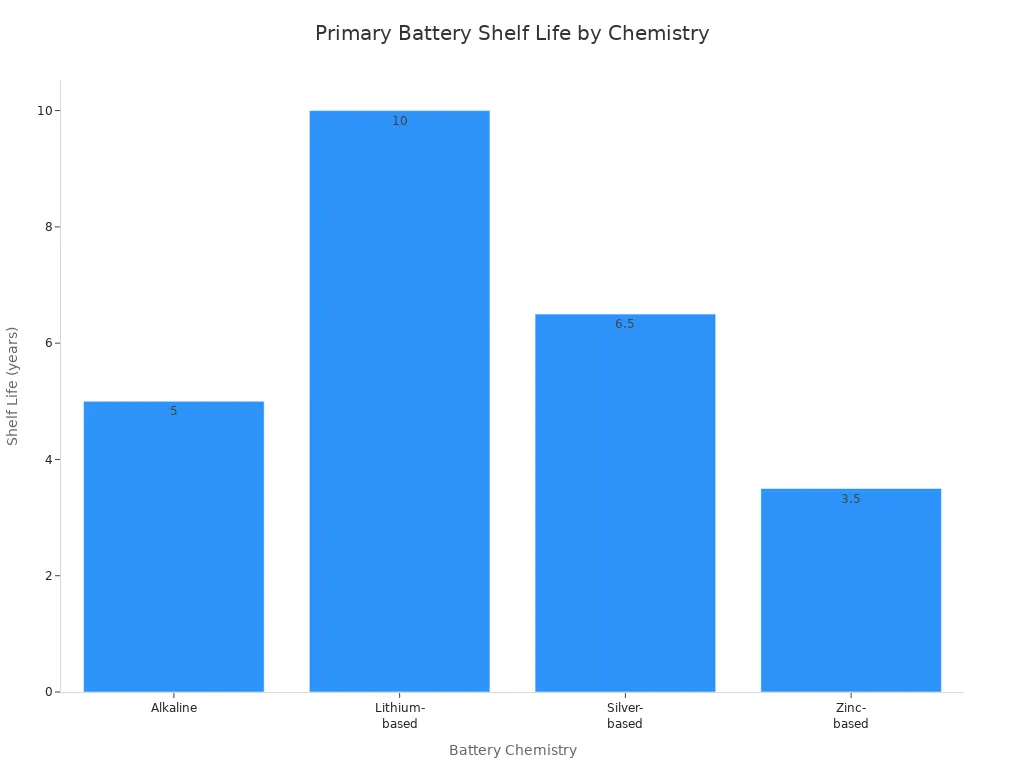
Note: Primary batteries remain popular for devices that require long shelf life and low to moderate power, such as remote controls and medical implants.
Secondary Battery Cells
Secondary battery cells, also known as rechargeable batteries, allow users to restore their charge through repeated cycles. These batteries include chemistries like lead-acid, nickel metal hydride, and lithium-ion. Rechargeable batteries have become essential in applications that demand frequent use and high energy output.
Key features of secondary batteries:
-
Higher initial cost but lower long-term expense
-
Superior performance for high discharge rates
|
Aspect |
Primary Batteries |
Secondary Batteries |
|---|---|---|
|
Rechargeability |
Single-use, cannot be recharged |
Rechargeable, can be reused multiple times |
|
Cycle Life |
Limited to one use |
Multiple charge-discharge cycles |
|
Cost-Effectiveness |
Lower initial cost, higher long-term cost |
Higher initial cost, more economical over time |
Nickel metal hydride (NiMH) batteries and lithium-ion batteries dominate the market for portable electronics, electric vehicles, and renewable energy storage. NiMH batteries offer good energy density and safety, while lithium-ion batteries provide high capacity and long cycle life. Lead-acid batteries remain common in automotive and backup power systems due to their robustness and affordability.
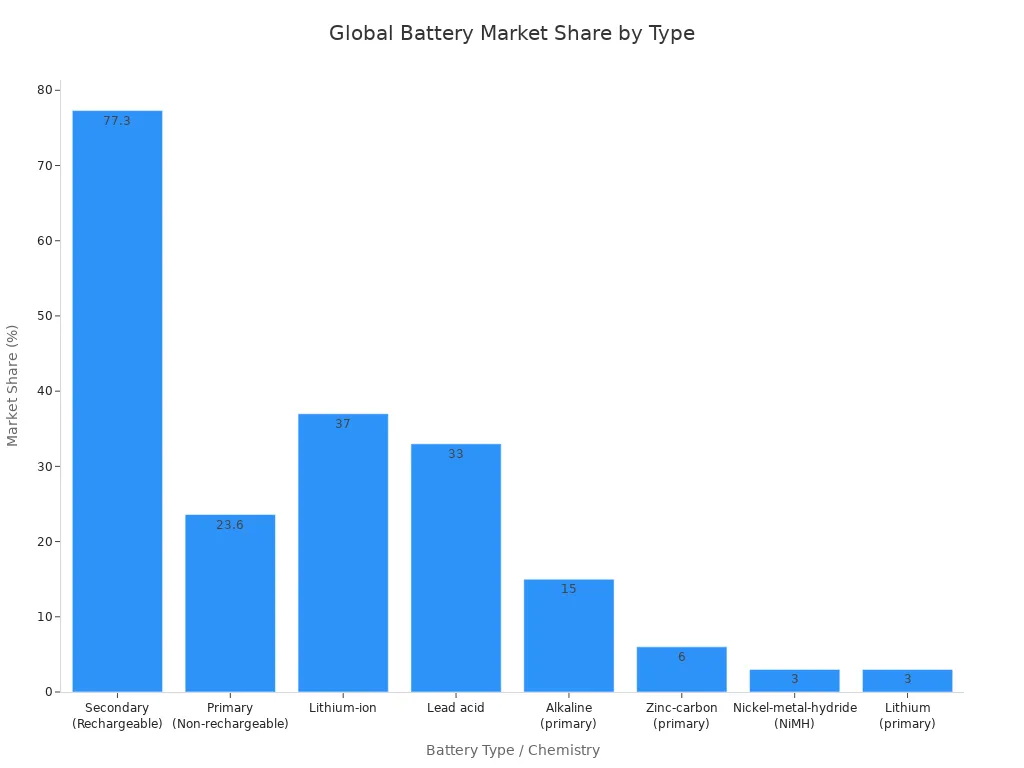
Secondary batteries now account for about 76.4% to 78.2% of global battery sales, reflecting their importance in modern technology.
Tip: Rechargeable batteries reduce waste and save money over time, making them ideal for high-use devices like smartphones, laptops, and electric vehicles.
Fuel and Reserve Cells
Fuel cell and reserve cells represent specialized types of batteries. A fuel cell generates electricity through a continuous supply of fuel, such as hydrogen, and an oxidizer, like oxygen. Unlike traditional batteries, a fuel cell does not store energy internally. Instead, it produces power as long as fuel flows into the system. Fuel cells achieve efficiencies between 40% and 60%, outperforming many combustion engines.
|
Aspect |
Advantages |
Limitations |
|---|---|---|
|
Clean Energy |
Produces electricity via electrochemical reaction, emitting only water vapor and heat |
N/A |
|
Efficiency |
High efficiency, direct conversion of fuel to electricity |
N/A |
|
Operation |
Quiet, suitable for noise-sensitive environments |
N/A |
|
Scalability |
Ranges from portable units to large power plants |
N/A |
|
Versatility |
Uses hydrogen, natural gas, or biogas |
Limited fuel availability, especially hydrogen |
|
Cost |
N/A |
Currently more expensive than traditional engines |
|
Infrastructure |
N/A |
Requires fuel production, storage, and distribution infrastructure |
|
Durability |
Reliable under proper conditions |
Durability affected by fuel purity and operating conditions |
|
Safety |
N/A |
Hydrogen is highly flammable, requiring careful handling |
Fuel cells power industrial equipment, backup systems, and military devices where clean, reliable, and long-lasting energy is critical. Reserve batteries, on the other hand, store a key component—usually the electrolyte—separately until activation. This design prevents self-discharge and chemical deterioration, making reserve batteries ideal for emergency and military applications such as missiles and torpedoes.
Note: Fuel cells and reserve batteries fill important roles in sectors that demand instant, reliable power or long-term storage without maintenance.
The different types of batteries—primary, secondary, fuel cell, and reserve—each offer unique benefits. Primary batteries provide convenience and long shelf life for low-drain devices. Secondary batteries deliver cost-effective, rechargeable power for frequent use. Fuel cells and reserve batteries support specialized applications in industry, military, and backup systems. Understanding these types of batteries and their chemistries, such as alkaline batteries, lithium cells, and nickel metal hydride, helps users make informed choices for any application.
Different Types of Battery Cells by Format

Battery cells come in several physical formats, each designed to meet specific performance, safety, and space requirements. The main formats include cylindrical, prismatic, pouch, and button or coin cells. Each format offers unique advantages and fits particular applications in electric vehicles, renewable energy storage, consumer electronics, and specialized devices.
Cylindrical Cells
Cylindrical cells have a round, tube-like shape and a metal casing. Manufacturers wind the electrodes into a spiral, known as a “jelly roll,” and insert them into a steel or aluminum cylinder. This design provides strong mechanical stability and efficient heat dissipation. Cylindrical cells are common in power tools, laptops, and electric vehicles such as the Tesla Model 3 and Model Y.
|
Battery Cell Type |
Defining Characteristics |
Construction Methods |
Typical Applications |
Advantages |
Disadvantages |
Special Considerations |
|---|---|---|---|---|---|---|
|
Cylindrical Cell |
Cylindrical shape, metal casing, long cycle life, high energy density |
Electrodes wound into a jelly roll, inserted into a metal cylinder casing, sealed with safety vents |
Electronics, power tools, electric vehicles |
Robust mechanical stability, good thermal management |
Larger volume for same capacity compared to prismatic/pouch |
Requires hi-pot testing and vacuuming during manufacturing |
Cylindrical cells like the 18650 and 21700 types have diameters of about 18 mm and 21 mm, respectively. Their lengths range from 65 mm to 70 mm. These cells use nickel-plated steel cans with thick bases for strength. Safety features include defined vent paths to release pressure if needed. The sealed design and robust casing help prevent swelling and leakage. Energy density typically ranges from 300 to 500 Wh/kg, and the cells can deliver high power output. Their shape allows for efficient cooling, which is important for high-performance applications.
Note: Cylindrical cells remain popular in electric vehicles and renewable energy storage because of their reliability, long cycle life, and established manufacturing processes.
Prismatic Cells
Prismatic cells have a rectangular, flat shape. Manufacturers stack or wind the electrodes and place them in a rigid aluminum housing. This format allows for better space utilization and flexible battery pack design. Prismatic cells are widely used in mobile phones, tablets, laptops, and electric vehicles from brands like Volkswagen and BMW.
|
Feature |
Prismatic Cells |
Cylindrical Cells |
|---|---|---|
|
Shape & Size |
Larger, rectangular prism shape |
Smaller, cylindrical shape |
|
Volumetric Energy Density |
Higher (600-700 Wh/L) |
Lower (500-600 Wh/L) |
|
Gravimetric Energy Density |
Lower (~200 Wh/kg) |
Higher (~260 Wh/kg) |
|
Power Density |
Moderate (1000-1200 W/kg) |
Higher (up to 1500 W/kg) |
|
Space Efficiency |
Better stacking and packing, more space-efficient |
Less efficient packing due to shape |
|
Applications |
EV battery packs, large energy storage systems |
Consumer electronics, power tools |
|
Manufacturing Trends |
Growing adoption in EVs and energy storage |
Remains dominant in cost-sensitive applications |
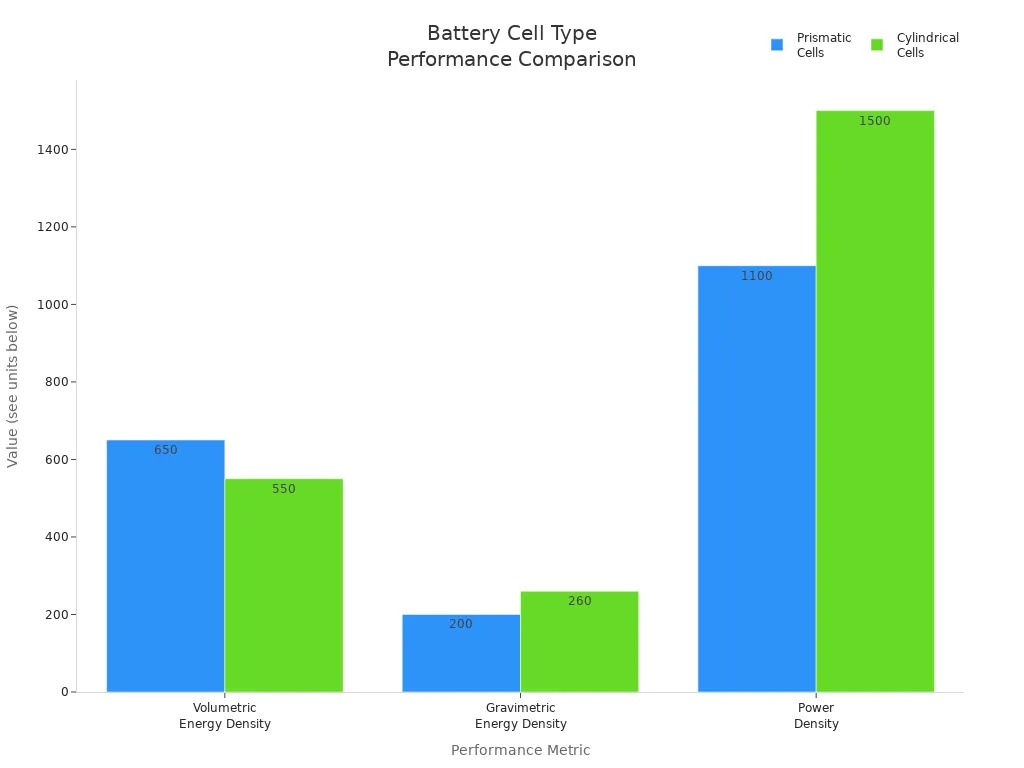
Prismatic cells offer higher volumetric energy density, which means they store more energy in the same space. Fewer cells are needed for the same capacity, reducing the number of electrical connections and simplifying battery management. However, prismatic cells can swell over time, sometimes increasing in thickness by up to 60%. Designers must allow space for this swelling to avoid damaging the device or compromising safety.
Tip: Prismatic cells are ideal for applications where space is limited and a flat battery pack is needed, such as in electric vehicles and large-scale energy storage.
Pouch Cells
Pouch cells use a flexible, lightweight aluminum-laminated film instead of a rigid metal casing. Manufacturers weld the electrodes to foil tabs and seal them inside a pouch. This design achieves the highest packaging efficiency, with up to 95% of the cell volume used for active materials. Pouch cells are common in smartphones, drones, military equipment, and some electric vehicles.
|
Advantages |
Details |
|---|---|
|
High packaging efficiency |
Up to 95%, maximizing space utilization |
|
Flexible design |
Customizable size and shape for various devices |
|
Lightweight |
Up to 40% lighter than metal-cased cells |
|
Low internal resistance |
Improves performance and reduces self-consumption |
|
Good cycle life |
Minimal capacity loss after thousands of cycles |
|
Enhanced safety |
Swells under pressure rather than exploding |
|
Smart BMS included |
Balances cells and protects against overcharge/discharge |
|
Disadvantages |
Details |
|---|---|
|
Fragile casing |
Easily damaged by mechanical stress |
|
Complex packaging |
Requires careful design for protection |
|
Medium-low capacity per cell |
Many cells needed in parallel for high-capacity packs |
|
Replacement difficulty |
Faulty cells cannot be replaced individually |
|
Manufacturing cost |
Relies on imported materials, increasing cost |
Pouch cells provide flexibility in battery pack design and reduce overall system weight. However, their soft casing makes them sensitive to pressure, vibration, and sharp edges. Swelling can occur, especially in high-capacity applications, so designers must avoid stacking and puncturing swollen cells.
Note: Pouch cells are gaining popularity in new energy vehicles and high-performance electronics due to their safety and energy density.
Button and Coin Cells
Button and coin cells are small, flat, and round. Most are primary (non-rechargeable) batteries, though some rechargeable types exist. Manufacturers layer the electrodes and electrolyte inside a metal casing and seal the cell. These cells power watches, hearing aids, medical implants, car keys, and small electronic devices.
|
Device/Application Types |
Typical Voltage Range (V) |
Capacity Range (mAh) |
|---|---|---|
|
Communication devices, sirens, small household |
1.5 to 12 |
0.001 to 950 |
|
Instruments, IC circuits, cameras, telecommunications, general-purpose electronics |
Typical: 0.0023 to 0.95 (Li-MnO2), up to 0.5+ (Li, NiMH) |
|
Battery Chemistry |
Voltage (V) |
Capacity Range (mAh) |
Applications |
|---|---|---|---|
|
Lithium Manganese Dioxide (ML Type) |
3 |
0.0023 to 0.045 |
General purpose, camera, telecommunications |
|
4.8 |
0.16 |
Communication, sirens, small household devices, instruments, IC circuits |
Button and coin cells offer very small size and long shelf life, making them ideal for low-power devices. Most have voltages between 1.5 and 12 volts and capacities from a few microamp-hours to nearly 1,000 mAh. Safety is important, as these cells can pose a risk if swallowed, especially by children.
Alert: Always keep button and coin cells out of reach of children to prevent accidental ingestion.
Each battery cell format serves a specific role in modern technology. Cylindrical, prismatic, pouch, and button or coin cells all contribute to the performance and safety of devices in sectors such as electric vehicles, renewable energy, consumer electronics, and medical equipment. Understanding these formats helps users select the best option for their application among the different types of batteries available today.
Lithium Ion Batteries and Other Chemistries

Lithium Ion Batteries
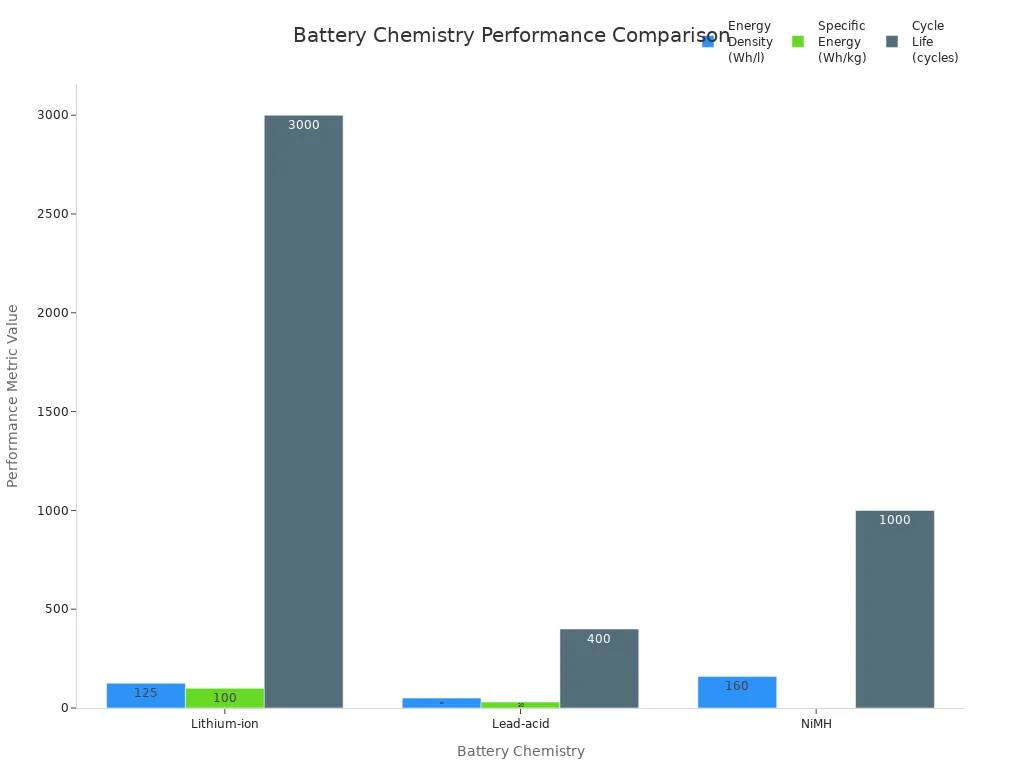
Lithium ion batteries have become the leading choice for powering electric vehicles, smartphones, laptops, and solar storage systems. These batteries use lithium cells with a carbon-based anode and various lithium-based cathodes, such as LCO, LFP, or NMC. The electrolyte contains flammable organic solvents, so a battery management system (BMS) is essential for safety. Lithium ion batteries deliver high energy density, ranging from 125 to over 600 Wh/l, and specific energy between 100 and 300 Wh/kg. Cycle life can reach up to 3,000 cycles at 80% depth of discharge. Most lithium cells provide a nominal voltage of 3.6 V, with stable power output and fast charging. Safety remains a top priority, as risks like thermal runaway and overheating exist. Manufacturers follow strict international standards and use advanced BMS to monitor voltage and temperature.
Lithium ion batteries dominate the global market. In 2024, the market size reached over USD 75 billion, with Asia-Pacific accounting for more than 44% of sales. China leads production, controlling about 75% of global lithium cell output. The automotive sector drives over half of the demand, and prices continue to fall as technology advances.
Tip: Always use lithium ion batteries with certified BMS and follow manufacturer guidelines for charging and storage.
Lead-Acid and NiMH Batteries
The lead acid battery remains a cost-effective solution for automotive starters, backup power, and industrial equipment. It uses lead dioxide and lead plates with a sulfuric acid electrolyte. While the upfront cost is low, the specific energy is only 30–40 Wh/kg, and cycle life is limited to 200–400 cycles. Lead acid batteries are heavy and contain toxic materials, so proper recycling is critical to prevent environmental harm.
NiMH batteries use nickel hydroxide and metal hydride alloys. They offer better energy density than lead acid, with values between 160 and 420 Wh/l. Cycle life ranges from 500 to 1,000 cycles. NiMH batteries are safer due to their non-flammable electrolyte and are common in hybrid vehicles, power tools, and some consumer electronics. Recycling NiMH batteries reduces environmental impact, especially compared to mining new metals.
|
Battery Chemistry |
Cathode Material |
Anode Material |
Electrolyte |
Energy Density (Wh/l) |
Specific Energy (Wh/kg) |
Cycle Life (cycles) |
Charge Efficiency |
Safety & Stability |
Cost |
Additional Notes |
|---|---|---|---|---|---|---|---|---|---|---|
|
Lithium-ion |
Various lithium-based cathodes |
Carbon-based (graphite) |
Flammable organic solvents |
125-600+ |
100-300 |
Up to 3000 |
>95% |
High temp. stability, BMS required |
High |
Lightweight, long cycle life |
|
Lead-acid |
Lead dioxide (PbO2) |
Lead |
Dilute sulfuric acid |
50-90 |
30-40 |
200-400 |
60-80% |
Lower temp. stability |
Low |
Heavy, cost-effective |
|
NiMH |
Nickel hydroxide |
Metal hydride alloys |
Alkaline potassium hydroxide |
160-420 |
N/A |
500-1000 |
Moderate |
Non-flammable, safer |
Moderate |
Good cycle life, less memory effect |
Note: Both lead acid and NiMH batteries require responsible recycling. Lead acid batteries have high recycling rates, while NiMH batteries are safer for the environment but still need proper disposal.
Emerging Battery Technologies
New battery chemistries are under development to improve performance, safety, and sustainability. Graphene batteries show promise with faster charging, higher energy capacity, and longer lifespan than traditional lithium cells. Companies like Samsung and Huawei invest in graphene research, aiming for commercial use in the next decade. Solid-state lithium batteries offer higher energy density and improved safety by replacing liquid electrolytes with solid materials. Sodium-ion and flow batteries, such as vanadium redox and zinc-bromide, provide alternatives for large-scale energy storage.
Advanced battery control systems play a key role in maximizing the benefits of these new chemistries. They optimize charging and discharging, extend battery life, and enhance safety. As technology evolves, emerging batteries will support the growing demand for electric vehicles, renewable energy, and portable electronics.
Alert: Always check for certified safety features and recycling options when choosing new battery technologies.
Choosing the Right Battery Cell
Key Factors to Consider
Selecting the right battery cell involves careful evaluation of several important factors. Each project or device has unique requirements, so understanding these aspects helps ensure optimal performance and safety.
-
Energy Density: High energy density allows devices to run longer between charges. Lithium-ion batteries excel in this area, making them ideal for compact electronics and electric vehicles.
-
Cycle Life: A long cycle life means the battery can be charged and discharged many times before losing capacity. Rechargeable batteries like lithium-ion and flow batteries offer excellent longevity, reducing replacement needs.
-
Safety: Safety features are critical. Look for built-in protections such as overcharge, over-discharge, and short-circuit prevention. Certifications like UL, CE, and IEC indicate that the battery meets strict safety standards.
-
Cost: Cost varies by chemistry. Lead-acid batteries are affordable but have lower performance. Rechargeable batteries may cost more upfront but save money over time.
-
Environmental Impact: Consider the recyclability of the battery and the presence of toxic materials. Some chemistries are easier to recycle and have less environmental impact.
-
Charge/Discharge Speed: Fast charging and discharging are important for dynamic applications. Lithium-ion batteries support rapid energy transfer.
-
Temperature Sensitivity: Batteries must operate safely within the expected temperature range. Some types require cooling systems or special management.
-
Physical Size and Shape: The battery must fit the device’s design. Options like cylindrical, prismatic, and pouch cells offer flexibility.
-
Supplier Reputation: Reliable suppliers provide quality assurance and support, reducing the risk of cell mismatch or early failure.
Tip: Always match the battery’s voltage and configuration to your device’s needs, and use a battery management system for added protection.
Matching Types to Applications
Different industries and devices require specific battery cell types. The choice depends on performance, safety, and operational demands.
-
Electric Vehicles (EVs): Lithium-ion rechargeable batteries are preferred for their high energy density and long cycle life. They enable longer driving ranges and faster charging.
-
Medical Devices: Devices like pacemakers and defibrillators rely on lithium-ion cells for reliable, long-lasting power. Safety and durability are essential in these applications.
-
Renewable Energy Storage: Lithium-ion and flow batteries store solar and wind energy efficiently. Their long cycle life and energy efficiency make them suitable for grid and home systems.
-
Consumer Electronics: Smartphones, laptops, and tablets use lithium-ion rechargeable batteries for compact size and high performance.
-
Industrial Equipment: Lead-acid batteries remain common for backup power and heavy machinery due to their low cost and reliability.
-
Low-Use Devices: Primary (non-rechargeable) batteries work well for remote controls and clocks, where long shelf life is more important than rechargeability.
|
Application Area |
Recommended Battery Type |
Key Considerations |
|---|---|---|
|
Electric Vehicles |
Lithium-ion rechargeable |
High energy density, cycle life |
|
Medical Devices |
Lithium-ion rechargeable |
Safety, reliability, long lifespan |
|
Renewable Energy |
Lithium-ion, flow rechargeable |
Cycle life, efficiency |
|
Consumer Electronics |
Lithium-ion rechargeable |
Size, fast charging |
|
Industrial Equipment |
Lead-acid, NiMH rechargeable |
Cost, robustness |
|
Low-Use Devices |
Primary (non-rechargeable) |
Shelf life, low maintenance |
Note: Avoid cell mismatch by using factory-sorted, high-quality cells and a robust battery management system. This practice improves reliability and extends battery life.
Battery cells come in many types and formats, each suited for specific uses.
-
Capacity-focused cells store more energy but cannot handle high-drain devices, while high-discharge cells power demanding equipment safely.
-
Prismatic cells offer higher energy per cell and fewer connections, while cylindrical cells provide faster discharge and greater stability.
Selecting the right cell prevents safety risks and ensures performance. Consulting battery experts and using diagnostic tools helps buyers match their needs, verify quality, and avoid costly mistakes.
What is the difference between a battery cell and a battery pack?
A battery cell is a single unit that stores and releases energy. A battery pack combines multiple cells to provide higher voltage or capacity for larger devices like electric vehicles or backup systems.
How long do lithium-ion battery cells usually last?
Most lithium-ion battery cells last between 2 and 5 years or up to 3,000 charge cycles. Proper care, such as avoiding extreme temperatures, helps extend their lifespan.
Which battery cell format works best for electric vehicles?
Prismatic and cylindrical cells both work well in electric vehicles. Prismatic cells save space, while cylindrical cells offer strong durability and easy cooling. Manufacturers choose based on design needs.
Are all battery cells safe for children’s devices?
|
Battery Type |
Safe for Children’s Devices? |
|---|---|
|
Alkaline |
Yes |
|
With protection |
|
|
No, choking hazard |
Always keep button and coin cells away from children to prevent accidental swallowing.
See Also
How To Utilize ADXL357BEZ For Motion Sensing And Stability
In-Depth Look At MC9S12DJ256MFUE Features For Vehicles
Detailed Instructions For Using AD620AN In Television Power
Ways Keep Booming Technology Sustains High Standards In Electronics
Enhancing Automotive Power Using NXP MC9S12XEP100 And MC9S12XS128


